Case studies
-

API Synthesis - Molecule 1
Task: Synthesis of 100mg of organic molecule with a highly complex 16 step synthetic route with very poor yield (<2% overall recovery) for use in GMP manufacture.
Outcome: Using a multi-targeted approach and investigating several reagents prepared in-house, the target molecule was delivered in high purity with shorter route and a much improved overall yield of 30%.
-

API Synthesis - Molecule 2
Task: Prepare Kg quantity of a complex drug candidate precursor cost effectively using a parallel synthesis for use in a GMP process.
Outcome: Improved the 12 step synthetic route requiring several elaborate protection/de-protection steps and coupling agents in a multi-batch synthesis process to give reproducible results. A full set of analytical methods was developed to support the chemistry and the product was delivered, complete with data on time and within budget.
-
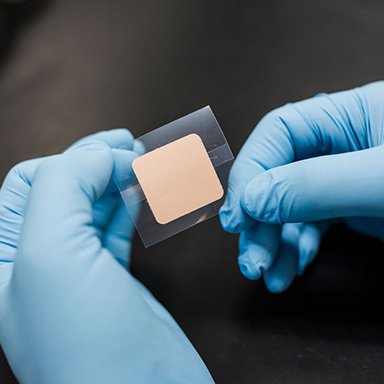
Transdermal Patch development and manufacture
We were approached by a multinational Pharmaceutical company seeking the development of a transdermal patch that had to bypass certain patents relating to the drug formulation, and still achieve drug release rates and pharmacokinetic profiles that matched the reference listed drug product. We developed the patch from scratch, produced batches for pilot clinical studies, and transferred the technology through to a CMO for commercial scale production, and provided all the requisite documentation to support an FDA submission. The client subsequently submitted an ANDA to the FDA.
-

TDS clinical batch manufacture
A client required a small-scale batch of transdermal patches for a pilot Pk study which contained a highly toxic and flammable solvent. We built an extractor system that allowed us to safely manufacture the patches using a bench-scale process within a 12 week period and the product was released for the clinical pilot study.
-

Topical gel manufacture for a PK study
A multinational client required a small-scale batch of topically applied gel to be produced in aluminium tubes and released for a clinical program. The manufacture process was partially complete and we optimised the manufacture process and manufactured the small scale batch and released for clinical use within a 10 week timeframe.
